
Chemistry, 09.12.2019 22:31 davidaagurto
Predict the sign of the entropy change, delta s, for each of the following reactions: the signs are either going to be pos or negativea) pb^2+(aq) + 2cl-(aq) > pbcl2(s)b) caco3(s) > cao(s) + co2 (g)c) 2nh3(g) > n2(g) + 3h2(g)d) p4(g) + 5o2(g) > p4o10(s)e) c4h8(g) + 6o2(g) > 4co2(g) + 4h2o(g)f) i2(s) > i2(g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1


Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1
You know the right answer?
Predict the sign of the entropy change, delta s, for each of the following reactions: the signs are...
Questions



Mathematics, 01.12.2020 01:00

Biology, 01.12.2020 01:00


Mathematics, 01.12.2020 01:00



Mathematics, 01.12.2020 01:00

Mathematics, 01.12.2020 01:00

Biology, 01.12.2020 01:00

Mathematics, 01.12.2020 01:00


English, 01.12.2020 01:00

Geography, 01.12.2020 01:00


Law, 01.12.2020 01:00


Mathematics, 01.12.2020 01:00

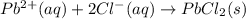 : negative
: negative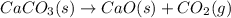 : positive
: positive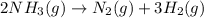 : positive.
: positive.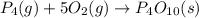 : negative
: negative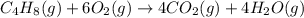 : positive.
: positive.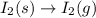 : positive.
: positive. is positive when randomness increases and
is positive when randomness increases and 



