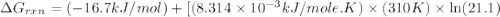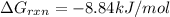Chemistry, 09.12.2019 23:31 monstergirl25
Consider the fructose-1,6-bisphosphatase reaction. calculate the free energy change if the ratio of the concentrations of the products to the concentrations of the reactants is 21.1 and the temperature is 37.0 ° c ? δ g ° ' for the reaction is − 16.7 kj/mol . δ g = kj / mol

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Consider the fructose-1,6-bisphosphatase reaction. calculate the free energy change if the ratio of...
Questions

Geography, 18.03.2021 02:20


English, 18.03.2021 02:20


Mathematics, 18.03.2021 02:20




Biology, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20






Advanced Placement (AP), 18.03.2021 02:20


Biology, 18.03.2021 02:20

English, 18.03.2021 02:20

Biology, 18.03.2021 02:20

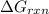 is -8.84 kJ/mol
is -8.84 kJ/mol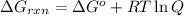 ............(1)
............(1)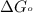 = standard Gibbs free energy = -16.7 kJ/mol
= standard Gibbs free energy = -16.7 kJ/mol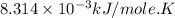
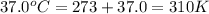
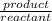 = 21.1
= 21.1