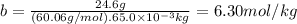Acertain substance condenses at a temperature of 123.3°c . but if a 65.0 gm sample of x is prepared with 24.6g of urea ((nh2)2co) dissolved in it, the sample is found to have a condensation point of 124.3°c instead.
1. calculate the molal boiling point elevation constant kb of x . round your answer to significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3
You know the right answer?
Acertain substance condenses at a temperature of 123.3°c . but if a 65.0 gm sample of x is prepared...
Questions



Mathematics, 01.04.2021 22:50


Biology, 01.04.2021 22:50


Mathematics, 01.04.2021 22:50

Mathematics, 01.04.2021 22:50




English, 01.04.2021 22:50


Biology, 01.04.2021 22:50

English, 01.04.2021 22:50


English, 01.04.2021 22:50


Mathematics, 01.04.2021 22:50

Computers and Technology, 01.04.2021 22:50