

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3


Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
Given that kp = 3.5 x 10-4 for the reaction 2 co(g) < => c(graphite) + co2(g), what is the pa...
Questions


History, 22.06.2019 00:00







Geography, 22.06.2019 00:00

History, 22.06.2019 00:00


History, 22.06.2019 00:00



Social Studies, 22.06.2019 00:00

History, 22.06.2019 00:00

Geography, 22.06.2019 00:00

History, 22.06.2019 00:00


Chemistry, 22.06.2019 00:00

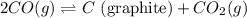
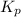 for above reaction follows:
for above reaction follows: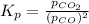
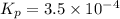
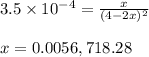
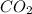 = 0.0056 atm
= 0.0056 atm

