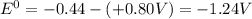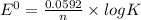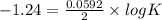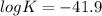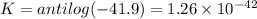Chemistry, 10.12.2019 01:31 sassycutie523
Eo cell = 0.0592 v n log(k) calculate the equilibrium constant for the following reaction at 25°c: 2ag(s) + fe2+(aq) ⇌ 2ag+(aq) + fe(s) enter your answer in scientific notation. k = × 10

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
Eo cell = 0.0592 v n log(k) calculate the equilibrium constant for the following reaction at 25°c:...
Questions

Physics, 27.04.2021 18:50




Mathematics, 27.04.2021 18:50


Mathematics, 27.04.2021 18:50

Mathematics, 27.04.2021 18:50

Mathematics, 27.04.2021 18:50






Mathematics, 27.04.2021 18:50



History, 27.04.2021 18:50

Mathematics, 27.04.2021 18:50

Mathematics, 27.04.2021 18:50

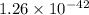
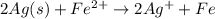
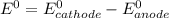
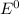 are standard reduction potentials.
are standard reduction potentials.
![E^0_{[Fe^{2+}/Fe]}=-0.44V](/tpl/images/0410/9384/59478.png)
![E^0_{[Ag^{+}/Ag]}=+0.80V](/tpl/images/0410/9384/76e17.png)
![E^0=E^0_{[Fe^{2+}/Fe]}- E^0_{[Ag^{+}/Ag]}](/tpl/images/0410/9384/81b1e.png)