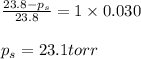Chemistry, 10.12.2019 01:31 romaguera06
Determine the vapor pressure of a solution at 25°c that contains 76.6 g of glucose (c6h12o6) in 250.0 ml of water. the vapor pressure of pure water at 25°c is 23.8 torr.70.8 torr7.29 torr72.9 torr22.9 torr23.1 torr

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1
You know the right answer?
Determine the vapor pressure of a solution at 25°c that contains 76.6 g of glucose (c6h12o6) in 250....
Questions
















Computers and Technology, 06.11.2019 23:31



Computers and Technology, 06.11.2019 23:31

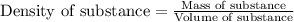
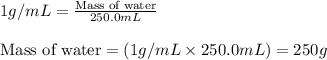
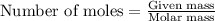 .....(1)
.....(1)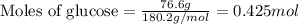
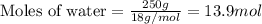
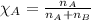
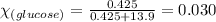
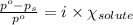
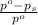 = relative lowering in vapor pressure
= relative lowering in vapor pressure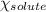 = mole fraction of solute = 0.030
= mole fraction of solute = 0.030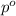 = vapor pressure of pure water = 23.8 torr
= vapor pressure of pure water = 23.8 torr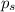 = vapor pressure of solution = ?
= vapor pressure of solution = ?