
Chemistry, 10.12.2019 03:31 ashtonbillups
Select the single best answer. a solid is hard, brittle, and electrically nonconducting. its melt (the liquid form of the substance) and an aqueous solution containing the substance conduct electricity. classify the solid. ionic covalent molecular metallic

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
You know the right answer?
Select the single best answer. a solid is hard, brittle, and electrically nonconducting. its melt (t...
Questions







Computers and Technology, 10.09.2019 02:30





Computers and Technology, 10.09.2019 02:30



Chemistry, 10.09.2019 02:30

Mathematics, 10.09.2019 02:30


Mathematics, 10.09.2019 02:30

Mathematics, 10.09.2019 02:30

Mathematics, 10.09.2019 02:30

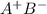 in solid state but in moleten state it will exist as
in solid state but in moleten state it will exist as 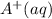 and
and  .
.

