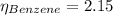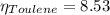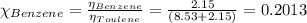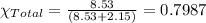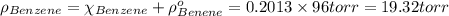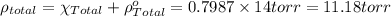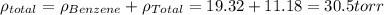In a mixture of volatile substances, the vapor pressure of the solution depends on the vapor pressure of both substances (solute and solvent). each component can evaporate, but its vapor pressure is lowered by the presence of the other substance following rault's law. the vapor pressure of the solvent is calculated using psolvent = xsolvent*posolvent and the vapor pressure of the solute is calculated using psolute = xsolute*posolute. the total vapor pressure can be calculated by dalton's law of partial pressures, ptotal = psolvent + psolute. at 25oc, the vapor pressure of pure benzene (c6h6, 78.11 g/mol) is 96 torr. at the same temperature, the vapor pressure of pure toluene (c7h8, 92.14 g/mol) is 14 torr. consider a solution containing 3.71 mol of benzene and 5.52 mol of toluene. calculate the vapor pressure above the solution. enter your answer in units of torr to three significant figures.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
In a mixture of volatile substances, the vapor pressure of the solution depends on the vapor pressur...
Questions

Biology, 27.09.2020 09:01



Mathematics, 27.09.2020 09:01

Health, 27.09.2020 09:01

Biology, 27.09.2020 09:01

English, 27.09.2020 09:01

Mathematics, 27.09.2020 09:01

French, 27.09.2020 09:01

English, 27.09.2020 09:01

Biology, 27.09.2020 09:01

History, 27.09.2020 09:01


Mathematics, 27.09.2020 09:01




Mathematics, 27.09.2020 09:01

Mathematics, 27.09.2020 09:01

Mathematics, 27.09.2020 09:01