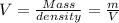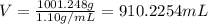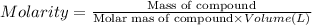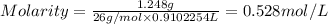Chemistry, 10.12.2019 04:31 ashanti0411
Asolution is 0.0480 m lif. what is the molarity of the solution if the density is 1.10 g/ml? 0.0480 m0.0436 m0.0528 m0.0417 m0.0441 m

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
Asolution is 0.0480 m lif. what is the molarity of the solution if the density is 1.10 g/ml? 0.0480...
Questions


Chemistry, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01




Mathematics, 20.10.2020 22:01


Engineering, 20.10.2020 22:01



Mathematics, 20.10.2020 22:01


Mathematics, 20.10.2020 22:01




History, 20.10.2020 22:01