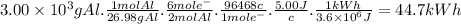The most useful ore of aluminum is bauxite, in which al is present as hydrated oxides, al2o3 * xh2o.
a) the number of kilowatt-hours of electricity required to produce 3.00kg of aluminum from electrolysis of compounds from bauxite is when the applied emf is 5.00 v.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3
You know the right answer?
The most useful ore of aluminum is bauxite, in which al is present as hydrated oxides, al2o3 * xh2o....
Questions

Biology, 22.01.2020 08:31

History, 22.01.2020 08:31


Biology, 22.01.2020 08:31

Physics, 22.01.2020 08:31

Mathematics, 22.01.2020 08:31



Biology, 22.01.2020 08:31


Mathematics, 22.01.2020 08:31

Mathematics, 22.01.2020 08:31


Mathematics, 22.01.2020 08:31



Mathematics, 22.01.2020 08:31

Mathematics, 22.01.2020 08:31