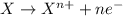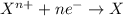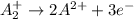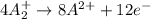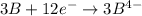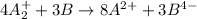Chemistry, 10.12.2019 04:31 summerhumphries3
Given two half reactions as follows: a2+ → 2 a2+ + 3 e− 4 e− + b → b4− what would you multiply each half-reaction by, to cancel out the electrons?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1


Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
Given two half reactions as follows: a2+ → 2 a2+ + 3 e− 4 e− + b → b4− what would you multiply each...
Questions


English, 13.10.2019 10:00

Mathematics, 13.10.2019 10:00



Mathematics, 13.10.2019 10:00




Biology, 13.10.2019 10:00


Biology, 13.10.2019 10:00


Mathematics, 13.10.2019 10:00

Mathematics, 13.10.2019 10:00



Mathematics, 13.10.2019 10:00

Mathematics, 13.10.2019 10:00

Advanced Placement (AP), 13.10.2019 10:00