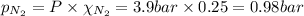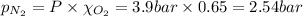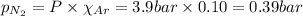Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
Amixture of n2, o2, and ar has mole fractions of 0.25, 0.65, and 0.10, respectively. what is the pre...
Questions



Biology, 05.11.2019 20:31



Social Studies, 05.11.2019 20:31

Social Studies, 05.11.2019 20:31


Physics, 05.11.2019 20:31


Mathematics, 05.11.2019 20:31

Mathematics, 05.11.2019 20:31



Spanish, 05.11.2019 20:31

Social Studies, 05.11.2019 20:31


Mathematics, 05.11.2019 20:31


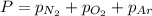
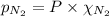
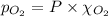
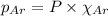
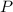 = total pressure = 3.9 bar
= total pressure = 3.9 bar 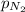 = partial pressure of nitrogen gas
= partial pressure of nitrogen gas 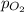 = partial pressure of oxygen gas
= partial pressure of oxygen gas 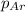 = partial pressure of argon gases
= partial pressure of argon gases 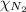 = Mole fraction of nitrogen gas = 0.25
= Mole fraction of nitrogen gas = 0.25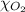 = Mole fraction of oxygen gas = 0.65
= Mole fraction of oxygen gas = 0.65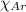 = Mole fraction of argon gases = 0.10
= Mole fraction of argon gases = 0.10