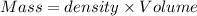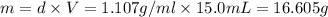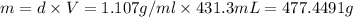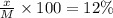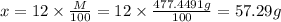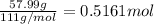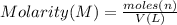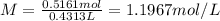A12.0 wt% solution of cacl2 (110.98 g/mol) has a density of 1.107 g/ml.
a) what is the mass (in milligrams) of a 15.0-ml solution of 12.0 wt% cacl2?
b) what is the mass (in grams) of cacl2 in 431.3 ml of a 12.0 wt% solution of cacl2?
c) what is the formal concentration of cacl2 (in molarity) of the 431.3-ml cacl2 solution described in part b?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
A12.0 wt% solution of cacl2 (110.98 g/mol) has a density of 1.107 g/ml.
a) what is the m...
a) what is the m...
Questions

Mathematics, 22.02.2021 23:20




History, 22.02.2021 23:20


History, 22.02.2021 23:20


SAT, 22.02.2021 23:20




Mathematics, 22.02.2021 23:20



History, 22.02.2021 23:20

Mathematics, 22.02.2021 23:20

Mathematics, 22.02.2021 23:20