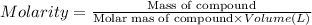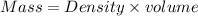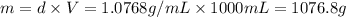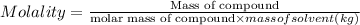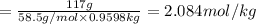Chemistry, 10.12.2019 05:31 hnsanders00
A2 m solution of nacl in water is at 20.0 oc. the density of the solution was measured to be 1.0768 g/ml at this temperature. calculate the molality of the salt solution. express your answer numerically to three significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2


Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
A2 m solution of nacl in water is at 20.0 oc. the density of the solution was measured to be 1.0768...
Questions

Computers and Technology, 24.12.2019 00:31

Computers and Technology, 24.12.2019 00:31

Computers and Technology, 24.12.2019 00:31








Computers and Technology, 24.12.2019 00:31




Biology, 24.12.2019 00:31

History, 24.12.2019 00:31

Biology, 24.12.2019 00:31

World Languages, 24.12.2019 00:31

History, 24.12.2019 00:31