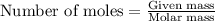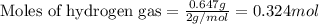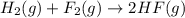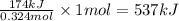The reaction of hydrogen(g) with fluorine(g) to form hydrogen fluoride(g) proceeds as follows: h2(g) + f2(g) 2 hf(g) when 0.647 grams of h2(g) react with sufficient f2(g) , 174 kj of energy are evolved . what is the value of h for the chemical equation given? δhrxn = kj

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 21.06.2019 23:30
Why do you suppose the structural polysaccharide cellulose does not contain branches? why do you suppose the structural polysaccharide cellulose does not contain branches? branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby decreasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby increasing the rigidity and strength of the microfibrils. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby increasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby decreasing the rigidity and strength of the microfibrils.
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

You know the right answer?
The reaction of hydrogen(g) with fluorine(g) to form hydrogen fluoride(g) proceeds as follows: h2(g...
Questions



Mathematics, 09.11.2020 16:40

Biology, 09.11.2020 16:40




Computers and Technology, 09.11.2020 16:40





Computers and Technology, 09.11.2020 16:40

Computers and Technology, 09.11.2020 16:40


Business, 09.11.2020 16:40

Physics, 09.11.2020 16:40



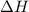 for the reaction will be -537 kJ
for the reaction will be -537 kJ