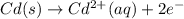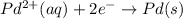Chemistry, 10.12.2019 06:31 antoinewill05
Avoltaic cell that uses the reaction pdcl2−4(aq)+cd(s)→pd(s)+4cl−(aq)+cd 2+(aq) has a measured standard cell potential of +1.03 v.
part a) write the two half-cell reactions. express your answer as a chemical equation. identify all of the phases in your answer.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2
You know the right answer?
Avoltaic cell that uses the reaction pdcl2−4(aq)+cd(s)→pd(s)+4cl−(aq)+cd 2+(aq) has a measured stand...
Questions



Chemistry, 14.01.2021 18:40

Mathematics, 14.01.2021 18:40

Chemistry, 14.01.2021 18:40



English, 14.01.2021 18:40







Computers and Technology, 14.01.2021 18:40



Chemistry, 14.01.2021 18:40

Mathematics, 14.01.2021 18:40

Mathematics, 14.01.2021 18:40