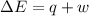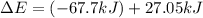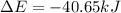Chemistry, 10.12.2019 06:31 wendelkristen
Consider a balloon filled with helium at the following conditions. 313 g he 1.00 atm 1910. l molar heat capacity = 20.8 j/degree c middot mol the temperature of this balloon is decreased by 41.6 degree c as the volume decreases to 1643 l with the pressure remaining constant. determine q, w, and delta e (in kj) for the compression of the balloon.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
Consider a balloon filled with helium at the following conditions. 313 g he 1.00 atm 1910. l molar h...
Questions



Mathematics, 10.06.2021 17:20

History, 10.06.2021 17:20

Mathematics, 10.06.2021 17:20

Arts, 10.06.2021 17:20

Biology, 10.06.2021 17:20

Mathematics, 10.06.2021 17:20


Mathematics, 10.06.2021 17:20

Mathematics, 10.06.2021 17:20



Mathematics, 10.06.2021 17:20

Mathematics, 10.06.2021 17:20

Mathematics, 10.06.2021 17:20

Computers and Technology, 10.06.2021 17:20


Mathematics, 10.06.2021 17:20

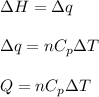
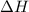 = change in enthalpy energy
= change in enthalpy energy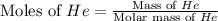
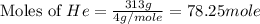
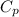 = heat capacity at constant pressure =
= heat capacity at constant pressure = 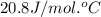
 = change in temperature =
= change in temperature = 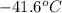
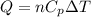
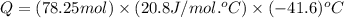
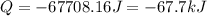
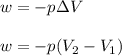
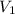 = initial volume = 1910 L
= initial volume = 1910 L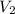 = final volume = 1643 L
= final volume = 1643 L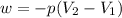
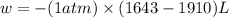
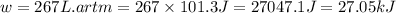
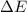 of the gas.
of the gas.