
Chemistry, 10.12.2019 07:31 CobyHageman
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, hcl ( aq ) , as described by the chemical equation
mno 2 ( s ) + 4 hcl ( aq ) ⟶ mncl 2 ( aq ) + 2 h 2 o ( l ) + cl 2 ( g )
how much mno 2 ( s ) should be added to excess hcl ( aq ) to obtain 255 ml cl 2 ( g ) at 25 °c and 795 torr ?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric ac...
Questions

English, 02.06.2021 21:10




Mathematics, 02.06.2021 21:10




Mathematics, 02.06.2021 21:10

Mathematics, 02.06.2021 21:10




Mathematics, 02.06.2021 21:10

English, 02.06.2021 21:10


Mathematics, 02.06.2021 21:10

Mathematics, 02.06.2021 21:10


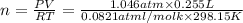
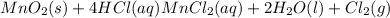
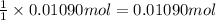 manganese dioxide
manganese dioxide

