
For the following reaction, 53.7 grams of iron(iii) oxide are allowed to react with 22.8 grams of aluminum. iron(iii) oxide (s) + aluminum (s) aluminum oxide (s) + iron (s) what is the maximum amount of aluminum oxide that can be formed? grams what is the formula for the limiting reagent? what amount of the excess reagent remains after the reaction is complete? grams

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
For the following reaction, 53.7 grams of iron(iii) oxide are allowed to react with 22.8 grams of al...
Questions


Computers and Technology, 09.12.2020 18:10

Chemistry, 09.12.2020 18:10





Mathematics, 09.12.2020 18:10








History, 09.12.2020 18:10

Mathematics, 09.12.2020 18:10



Mathematics, 09.12.2020 18:10

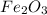 .
.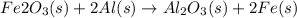
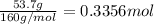
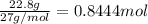
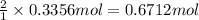 of aluminum.
of aluminum.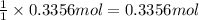 of aluminum oxide
of aluminum oxide

