
An 8.8? g ice cube is placed into 250g of water. calculate the temperature change in the water upon the complete melting of the ice. assume that all of the energy required to melt the ice comes from the water. express your answer in terms of the initial temperature of water, t.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
An 8.8? g ice cube is placed into 250g of water. calculate the temperature change in the water upon...
Questions



Health, 29.08.2019 19:50



Physics, 29.08.2019 19:50

Mathematics, 29.08.2019 19:50



Biology, 29.08.2019 19:50



English, 29.08.2019 19:50

English, 29.08.2019 19:50

Mathematics, 29.08.2019 19:50


Mathematics, 29.08.2019 19:50


Mathematics, 29.08.2019 19:50

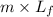
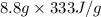
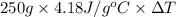 = 2930.4 J
= 2930.4 J  = 2.8°C
= 2.8°C

