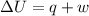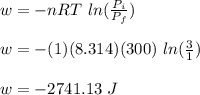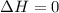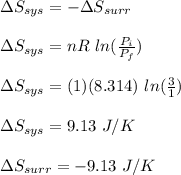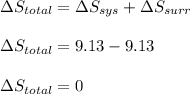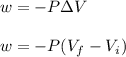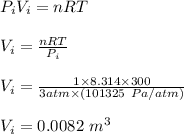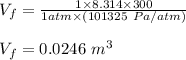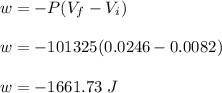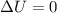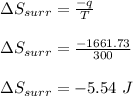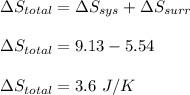Asample consisting of 1.00 mol of perfect gas molecules at 27 °c is expanded isothermally from an initial pressure of 3.00 atm to a final pressure of 1.00 atm in two ways: (a) reversibly, and (b) against a constant external pressure of 1.00 atm. evaluate q, w, δu, δh, δs, δssurr, and δstot in each case.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2


Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
Asample consisting of 1.00 mol of perfect gas molecules at 27 °c is expanded isothermally from an in...
Questions


Mathematics, 05.11.2020 21:40

Arts, 05.11.2020 21:40



Biology, 05.11.2020 21:40


Mathematics, 05.11.2020 21:40


Mathematics, 05.11.2020 21:40


World Languages, 05.11.2020 21:40



Arts, 05.11.2020 21:40


Mathematics, 05.11.2020 21:40


History, 05.11.2020 21:40

Social Studies, 05.11.2020 21:40

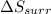 is -9.13 J/K, the entropy change of the system,
is -9.13 J/K, the entropy change of the system, 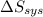 is 9.13 J/K, and the total entropy change,
is 9.13 J/K, and the total entropy change, 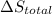 is 0.
is 0.