
Chemistry, 10.12.2019 21:31 keasiabradley
Water's unique properties, including its high heat capacity, high density, high boiling point and a solid phase that is less dense than its liquid phase, can best be attributed to:
a) its formula, h2o.
b) the covalent oxygen-hydrogen bonds in the molecule.
c) the shape of the molecule.
d) the polarity of the molecule and hydrogen bonding between the molecules.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 10:20
El amoniaco y el fluor reaccionan para formar tetrafluoruro de dinitrogeno y fluoruro de hidrogeno. segun la reaccion: nh3 + f2 ⇒ n2f4 + hf si reaccionan 5 gramos de amoniaco y 20 gramos de fuor, ¿cuantos gramos de fluoruro de hidrogeno se producen?
Answers: 2
You know the right answer?
Water's unique properties, including its high heat capacity, high density, high boiling point and a...
Questions


Chemistry, 11.12.2019 22:31




Social Studies, 11.12.2019 22:31














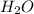 and due to the difference in electronegativity of hydrogen and oxygen atom there is formation of partial positive charge on hydrogen and partial negative charge on oxygen atom.
and due to the difference in electronegativity of hydrogen and oxygen atom there is formation of partial positive charge on hydrogen and partial negative charge on oxygen atom.

