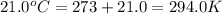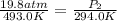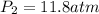Chemistry, 10.12.2019 21:31 payshencec21
A20.6-l sample of "pure" air is collected in greenland at a temperature of 220.0°c and a pressure of 1.01 atm and is forced into a 1.05-l bottle for shipment to europe for analysis.
(a) compute the pressure inside the bottle just after it is filled.
(b) compute the pressure inside the bottle as it is opened in the 21.0°c comfort of the european laboratory.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2


Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
A20.6-l sample of "pure" air is collected in greenland at a temperature of 220.0°c and a pressure of...
Questions

Computers and Technology, 24.03.2021 22:10

Biology, 24.03.2021 22:10


Mathematics, 24.03.2021 22:10



Mathematics, 24.03.2021 22:10



Biology, 24.03.2021 22:10


Mathematics, 24.03.2021 22:10

Physics, 24.03.2021 22:10

Mathematics, 24.03.2021 22:10



Mathematics, 24.03.2021 22:10

German, 24.03.2021 22:10

Computers and Technology, 24.03.2021 22:10

English, 24.03.2021 22:10

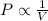
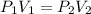
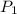 = initial pressure of gas = 1.01 atm
= initial pressure of gas = 1.01 atm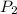 = final pressure of gas = ?
= final pressure of gas = ?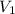 = initial volume of gas = 20.6 L
= initial volume of gas = 20.6 L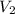 = final volume of gas = 1.05 L
= final volume of gas = 1.05 L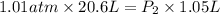
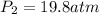
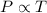
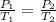
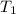 = initial temperature of gas =
= initial temperature of gas = 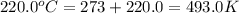
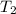 = final temperature of gas =
= final temperature of gas =