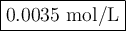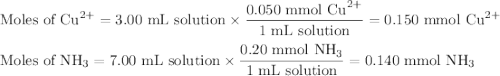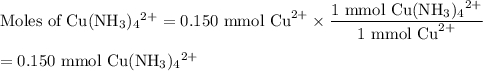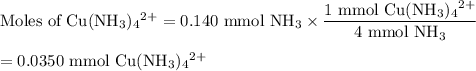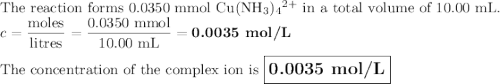Chemistry, 11.12.2019 00:31 diamondgdm
Astudent mixes in a test tube 3.00ml of 0.050m cuso4with 7.00ml of 0.20m nh3/nh41 . the solution becomes a deep blue color. assuming all the cu2 is complexed with nh3to form the [cu(nh3)4]2 ion, determine the concentration of the complex in the solution.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
Astudent mixes in a test tube 3.00ml of 0.050m cuso4with 7.00ml of 0.20m nh3/nh41 . the solution bec...
Questions

Mathematics, 25.05.2021 17:00




Mathematics, 25.05.2021 17:00



Mathematics, 25.05.2021 17:00

Mathematics, 25.05.2021 17:00




History, 25.05.2021 17:00

Mathematics, 25.05.2021 17:00



History, 25.05.2021 17:00


Social Studies, 25.05.2021 17:00