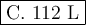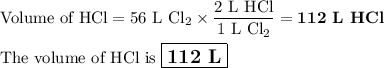Consider this reaction:
h2 (g) + cl2 (g) → 2 hcl (g)
how many liters of hcl are produce...

Chemistry, 11.12.2019 02:31 yashirachevalier
Consider this reaction:
h2 (g) + cl2 (g) → 2 hcl (g)
how many liters of hcl are produced when 56 l of chlorine are reacted with excess
hydrogen?
(one mole of any gas occupies 22.4 l under certain conditions of temperature and
pressure. assume those conditions for this question.)
a. 22.4l
b. 56 l
c. 112
d. 224 l

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
Questions

Mathematics, 31.08.2019 08:00

English, 31.08.2019 08:00


Geography, 31.08.2019 08:00



Mathematics, 31.08.2019 08:00



Biology, 31.08.2019 08:00

History, 31.08.2019 08:00

History, 31.08.2019 08:00

Mathematics, 31.08.2019 08:00

Biology, 31.08.2019 08:00



Physics, 31.08.2019 08:10