Chemistry, 11.12.2019 02:31 sydthekid25
A1.0 g sample of hydrogen reacts completely with 19.0 g of fluorine to form a compound of hydrogen and fluorine. a. what is the percent by mass of each element in the compound? b. what mass of hydrogen would be present in a 50 g sample of this compound? c. justify your answer to b.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2
You know the right answer?
A1.0 g sample of hydrogen reacts completely with 19.0 g of fluorine to form a compound of hydrogen a...
Questions


Mathematics, 01.10.2019 17:30


Mathematics, 01.10.2019 17:30


History, 01.10.2019 17:30


Mathematics, 01.10.2019 17:30




Biology, 01.10.2019 17:30


Mathematics, 01.10.2019 17:30



Social Studies, 01.10.2019 17:30


Chemistry, 01.10.2019 17:30

Physics, 01.10.2019 17:30

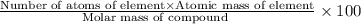
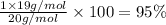
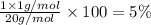
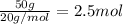
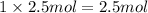 of hydrogen atom.
of hydrogen atom.