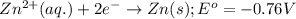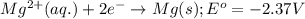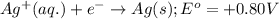Consider the following standard reduction potentials: zn2+(aq) + 2 e− latex: \longrightarrow⟶ zn(s) eo = −0.76 v mg2+(aq) + 2 e− latex: \longrightarrow⟶ mg(s) eo = −2.37 v ag+(aq) + e− latex: \longrightarrow⟶ ag(s) eo = +0.80 v which is the strongest oxidizing agent? group of answer choices

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2


Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3
You know the right answer?
Consider the following standard reduction potentials: zn2+(aq) + 2 e− latex: \longrightarrow⟶ zn(s...
Questions

Mathematics, 08.01.2021 03:50


Mathematics, 08.01.2021 03:50




Social Studies, 08.01.2021 03:50

Mathematics, 08.01.2021 03:50

Social Studies, 08.01.2021 03:50



Mathematics, 08.01.2021 03:50

History, 08.01.2021 03:50


Mathematics, 08.01.2021 03:50

English, 08.01.2021 03:50



Mathematics, 08.01.2021 03:50

 potential will always get reduced and will undergo reduction reaction easily.
potential will always get reduced and will undergo reduction reaction easily.