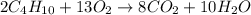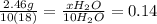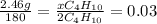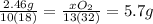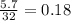The combustion of a sample of butane, c4h10, produced 2.46 grams of water.
2c4h10 + 13o2 ->...

The combustion of a sample of butane, c4h10, produced 2.46 grams of water.
2c4h10 + 13o2 -> 8co2 + 10h20
(a) how many moles of water formed? (b) how many moles of butane burned?
(c) how many grams of butane burned?
(d) how much oxygen was used up in moles? and in grams?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1

Chemistry, 23.06.2019 08:30
Kelly has come up with an explanation for why her sister is sometimes in a good mood and other times in a bad mood. she speculates that it is based on the hours of sleep her sister got the previous night. this explanation for her sister's behaviors is an example of a(n)
Answers: 3
You know the right answer?
Questions



Mathematics, 19.07.2019 22:50





Biology, 19.07.2019 22:50

Biology, 19.07.2019 22:50





Mathematics, 19.07.2019 22:50

History, 19.07.2019 22:50

Physics, 19.07.2019 22:50

Biology, 19.07.2019 22:50



Mathematics, 19.07.2019 22:50