
A41.1 g sample of solid co2 (dry ice) is added to a container at a temperature of 100 k with a volume of 3.4 l. a. if the container is evacuated (all of the gas removed), sealed, and then allowed to warm to room temperature t = 298 k so that all of the solid co2 is converted to a gas, what is the pressure inside the container?

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2
You know the right answer?
A41.1 g sample of solid co2 (dry ice) is added to a container at a temperature of 100 k with a volum...
Questions



Mathematics, 08.09.2021 07:50

Chemistry, 08.09.2021 07:50

Mathematics, 08.09.2021 07:50



History, 08.09.2021 07:50


Social Studies, 08.09.2021 07:50

Mathematics, 08.09.2021 07:50

Biology, 08.09.2021 07:50

French, 08.09.2021 07:50

Mathematics, 08.09.2021 07:50



Physics, 08.09.2021 07:50


Mathematics, 08.09.2021 07:50

Physics, 08.09.2021 07:50

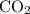 :
: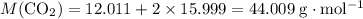 .
.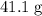 sample of
sample of 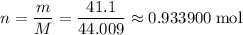 .
.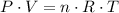 ,
, 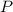 is the pressure inside the container.
is the pressure inside the container.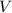 is the volume of the container.
is the volume of the container.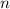 is the number of moles of particles (molecules, or atoms in case of noble gases) in the gas.
is the number of moles of particles (molecules, or atoms in case of noble gases) in the gas.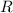 is the ideal gas constant.
is the ideal gas constant. 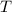 is the absolute temperature of the gas.
is the absolute temperature of the gas.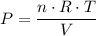 .
.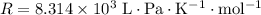 .
.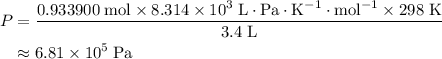 .
.

