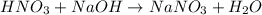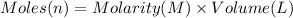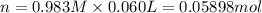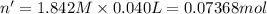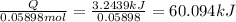Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
In a calorimeter, you combine 60 ml of a 0.983 m hno3 solution with 40 ml of a 1.842 m naoh solution...
Questions

Mathematics, 04.07.2021 01:00



Social Studies, 04.07.2021 01:00


Computers and Technology, 04.07.2021 01:00



Mathematics, 04.07.2021 01:00





Mathematics, 04.07.2021 01:00


Chemistry, 04.07.2021 01:00

Mathematics, 04.07.2021 01:00


Chemistry, 04.07.2021 01:10