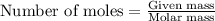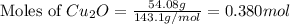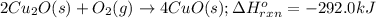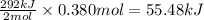Chemistry, 11.12.2019 05:31 Lydiac8715
The oxidation of copper(i) oxide, cu 2 o ( s ) , to copper(ii) oxide, cuo ( s ) , is an exothermic process. 2 cu 2 o ( s ) + o 2 ( g ) ⟶ 4 cuo ( s ) δ h ∘ rxn = − 292.0 kj mol calculate the energy released as heat when 54.08 g cu 2 o ( s ) undergo oxidation at constant pressure.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1

Chemistry, 23.06.2019 04:00
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1
You know the right answer?
The oxidation of copper(i) oxide, cu 2 o ( s ) , to copper(ii) oxide, cuo ( s ) , is an exothermic p...
Questions

English, 05.11.2020 20:30


Chemistry, 05.11.2020 20:30

Mathematics, 05.11.2020 20:30

Mathematics, 05.11.2020 20:30


Mathematics, 05.11.2020 20:30

Physics, 05.11.2020 20:30


Mathematics, 05.11.2020 20:30

Mathematics, 05.11.2020 20:30



Mathematics, 05.11.2020 20:30

Geography, 05.11.2020 20:30


Health, 05.11.2020 20:30

Arts, 05.11.2020 20:30


Mathematics, 05.11.2020 20:30

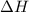 for the reaction will be -55.48 kJ
for the reaction will be -55.48 kJ