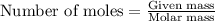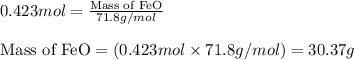Chemistry, 11.12.2019 05:31 nichelle2807
What mass of iron(ii) oxide must be used in the reaction given by the equation below to release 44.7 kj? 6feo(s) + o2(g) => 2fe3o4(s) δh° = -635 kj calculate your answer in g. enter it with two decimal places and no units.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
What mass of iron(ii) oxide must be used in the reaction given by the equation below to release 44.7...
Questions

History, 03.11.2020 04:30

Geography, 03.11.2020 04:30

Spanish, 03.11.2020 04:30

Mathematics, 03.11.2020 04:30

Mathematics, 03.11.2020 04:30


Chemistry, 03.11.2020 04:30



Mathematics, 03.11.2020 04:30



Mathematics, 03.11.2020 04:30




Mathematics, 03.11.2020 04:30

English, 03.11.2020 04:30

Mathematics, 03.11.2020 04:30

Social Studies, 03.11.2020 04:30

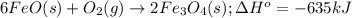
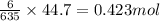 of iron (II) oxide is reacted.
of iron (II) oxide is reacted.