
Chemistry, 11.12.2019 05:31 daemonacoster
Arrange the following solutions in order of decreasing freezing point: 0.20 m baf2, 0.15 m c6h12, 0.10 m crbr3, 0.15 m ch3ch2ch2cooh, 0.35 m kbr. (enter just the formulas in your answer. use the appropriate < , =, or > symbol to separate substances in the list.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2
You know the right answer?
Arrange the following solutions in order of decreasing freezing point: 0.20 m baf2, 0.15 m c6h12, 0...
Questions

Mathematics, 06.10.2020 15:01



Social Studies, 06.10.2020 15:01

Mathematics, 06.10.2020 15:01


English, 06.10.2020 15:01



Mathematics, 06.10.2020 15:01




Mathematics, 06.10.2020 15:01


Mathematics, 06.10.2020 15:01

Mathematics, 06.10.2020 15:01

History, 06.10.2020 15:01


English, 06.10.2020 15:01

 > 0.20 m
> 0.20 m  > 0.10 m
> 0.10 m  > 0.15 m
> 0.15 m  > 0.15 m
> 0.15 m 
 and
and 
 and
and 
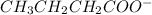 and
and  .
. and
and  .
.

