
The 235u isotope (atomic mass = 235.00) undergoes fission when bombarded with neutrons. however, its natural abundance is only 0.72 percent. to separate it from the more abundant 238u isotope (atomic mass = 238.00), uranium is first converted to uf6, which is easily vaporized above room temperature. the mixture of 235uf6 and 238uf6 gases is then subjected to many stages of effusion. calculate how much more quickly 235uf6 effuses than 238uf6. give the answer as the ratio of rates of 235uf6 to 238uf6 to four decimal places.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2


Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1
You know the right answer?
The 235u isotope (atomic mass = 235.00) undergoes fission when bombarded with neutrons. however, its...
Questions

History, 05.05.2020 04:47




Computers and Technology, 05.05.2020 04:47



Arts, 05.05.2020 04:47

English, 05.05.2020 04:47




History, 05.05.2020 04:47

Health, 05.05.2020 04:47

History, 05.05.2020 04:47


Mathematics, 05.05.2020 04:47



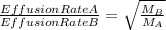
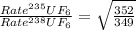 = 1.0043
= 1.0043

