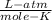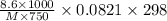Chemistry, 11.12.2019 06:31 nails4life324
Lysine is an amino acid that is an essential part of nutrition but which is not synthesized by the human body. what is the molar mass of lysine if 750.0 ml of a solution containing 8.60 g of lysine has an osmotic pressure of 1.918 atm? temperature = 25.0°c

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

You know the right answer?
Lysine is an amino acid that is an essential part of nutrition but which is not synthesized by the h...
Questions



History, 18.02.2020 05:00

Mathematics, 18.02.2020 05:00





Mathematics, 18.02.2020 05:00



Mathematics, 18.02.2020 05:00



History, 18.02.2020 05:01





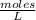 (Molarity).
(Molarity).