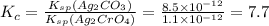Chemistry, 11.12.2019 19:31 mariahrpoulin1511
The value of the solubility product constant for ag2co3 is 8.5 × 10‒12 and that of ag2cro4 is 1.1 × 10‒12. from this data, what is the value of kc for the reaction, ag2co3(s) + cro42‒(aq) → ag2cro4(s) + co32‒(aq) a) 9.6 × 10‒12 b) 7.7 c) 1.1 × 1023 d) 1.3 × 10‒1 e) 9.4 × 10‒24

Answers: 2
Another question on Chemistry



Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
The value of the solubility product constant for ag2co3 is 8.5 × 10‒12 and that of ag2cro4 is 1.1 ×...
Questions


Mathematics, 24.02.2020 22:51

Biology, 24.02.2020 22:52



English, 24.02.2020 22:52



Mathematics, 24.02.2020 22:53


Law, 24.02.2020 22:53

Mathematics, 24.02.2020 22:53

Mathematics, 24.02.2020 22:53


Mathematics, 24.02.2020 22:54



Mathematics, 24.02.2020 22:54

Mathematics, 24.02.2020 22:54

Mathematics, 24.02.2020 22:54

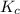 for the given reaction is 7.7
for the given reaction is 7.7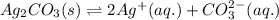
![K_{sp}(Ag_{2}CO_{3})=[Ag^{+}]^{2}[CO_{3}^{2-}]](/tpl/images/0413/8617/b8832.png)
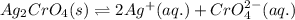
![K_{sp}(Ag_{2}CrO_{4})=[Ag^{+}]^{2}[CrO_{4}^{2-}]](/tpl/images/0413/8617/31f53.png)
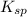 represents solubility product
represents solubility product![K_{c}=\frac{[CO_{3}^{2-}]}{[CrO_{4}^{2-}]}](/tpl/images/0413/8617/fea9e.png) (concentration of pure solids remain constant during reaction. Hence their concentration is taken as 1 to exclude them from equilibrium constant expression)
(concentration of pure solids remain constant during reaction. Hence their concentration is taken as 1 to exclude them from equilibrium constant expression)![K_{c}=\frac{[Ag^{+}]^{2}[CO_{3}^{2-}]}{[Ag^{+}]^{2}[CrO_{4}^{2-}]}](/tpl/images/0413/8617/3fc1b.png)