
Chemistry, 11.12.2019 20:31 brainist71
Consider the following equilibrium at 970 k for the dissociation of molecular iodine into atoms of iodine. i2(g) equilibrium reaction arrow 2 i(g); kc = 1.35 ✕ 10−3 suppose this reaction is initiated in a 3.7 l container with 0.063 mol i2 at 970 k. calculate the concentrations of i2 and i at equilibrium.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
Consider the following equilibrium at 970 k for the dissociation of molecular iodine into atoms of i...
Questions

Mathematics, 26.10.2020 05:40



Biology, 26.10.2020 05:40


Biology, 26.10.2020 05:40

Biology, 26.10.2020 05:40




History, 26.10.2020 05:40

Mathematics, 26.10.2020 05:40


Mathematics, 26.10.2020 05:40

History, 26.10.2020 05:40


Biology, 26.10.2020 05:40

Chemistry, 26.10.2020 05:40


History, 26.10.2020 05:40

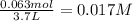
![Kc=1.35 \times 10^{-3} =\frac{[I]^{2} }{[I_{2}]} =\frac{(2x)^{2} }{(0.017-x)} \\4x^{2} +1.35 \times 10^{-3}x - 2.3 \times 10^{-5}](/tpl/images/0413/9700/35688.png)



