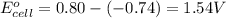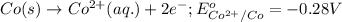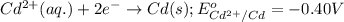Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

You know the right answer?
Which of the following redox reactions do you expect to occur spontaneously in the forward direction...
Questions

English, 25.01.2021 04:10

Chemistry, 25.01.2021 04:10

Business, 25.01.2021 04:10

Mathematics, 25.01.2021 04:10









Mathematics, 25.01.2021 04:10


Chemistry, 25.01.2021 04:10




Mathematics, 25.01.2021 04:10

English, 25.01.2021 04:10

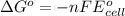
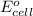 of the reaction, we use the equation:
of the reaction, we use the equation: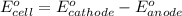 .......(1)
.......(1)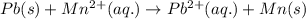
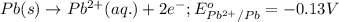
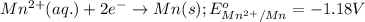
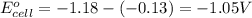
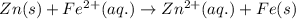

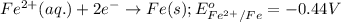
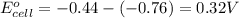
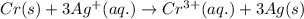
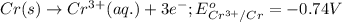
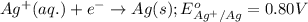 ( × 3)
( × 3)