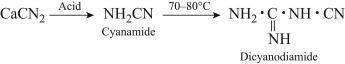Chemistry, 11.12.2019 21:31 alarconanais07
Cac2 is prepared at high temperature from limestone and charcoal. the first reaction in this process is roasting calcium carbonate at 800 oc to give calcium oxide and a gaseous product. the second involves the metal oxide produced (in the first) with carbon at 2000 oc to give the desired product and carbon monoxide
write the two balanced chemical equations for the reactions:
eqn. 1
eqn. 2
because of the energy costs of these processes, commercial acetylene production using cac2 is more costly than obtaining it from the decomposition reaction of natural gas (methane) under controlled conditions. however, cac2 is important in the manufacture of plastics. at elevated temperatures (1000 oc) it reacts directly with nitrogen gas to give a product called calcium cyanamide and carbon.
write the balanced chemical equation:
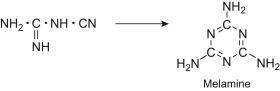
the compound is one of the starting materials in a four step industrial synthesis of melamine polymers, which are used to make light weight, heat resistant tableware.
write chemical equations in the four step reactions towards the manufacture of melamine polymers

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1
You know the right answer?
Cac2 is prepared at high temperature from limestone and charcoal. the first reaction in this process...
Questions

History, 11.05.2021 04:10

Mathematics, 11.05.2021 04:10





Mathematics, 11.05.2021 04:10

Biology, 11.05.2021 04:10

Health, 11.05.2021 04:10

Mathematics, 11.05.2021 04:10

Mathematics, 11.05.2021 04:10



Advanced Placement (AP), 11.05.2021 04:10



Mathematics, 11.05.2021 04:10