
Chemistry, 11.12.2019 21:31 keniaguevara32
Calculate δhrxn for the following reaction: ch4(g)+2cl2(g)→ch2cl2(g)+2hcl(g) use the following reactions and given δh values. ch4(g)+cl2(g)→ch3cl(g)+hcl(g), δh=−99.60 kj ch3cl(g)+cl2(g)→ch2cl2(g)+hcl(g), δh=−105.8 kj express your answer to four significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Calculate δhrxn for the following reaction: ch4(g)+2cl2(g)→ch2cl2(g)+2hcl(g) use the following reac...
Questions


Biology, 31.01.2020 06:48


History, 31.01.2020 06:48





Mathematics, 31.01.2020 06:49



Chemistry, 31.01.2020 06:49

Physics, 31.01.2020 06:49

Mathematics, 31.01.2020 06:49

Social Studies, 31.01.2020 06:49

Physics, 31.01.2020 06:49


Mathematics, 31.01.2020 06:49


Mathematics, 31.01.2020 06:49

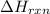 for the reaction is -205.4 kJ.
for the reaction is -205.4 kJ.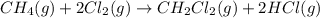
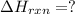
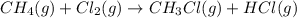
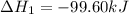
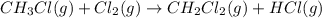
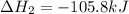
![\Delta H_{rxn}=[1\times \Delta H_1]+[1\times \Delta H_2]](/tpl/images/0414/0614/6e774.png)
![\Delta H_{rxn}=[(1\times (-99.60))+(1\times (-105.8))]=-205.4kJ](/tpl/images/0414/0614/3a575.png)


