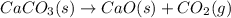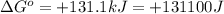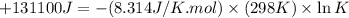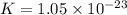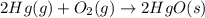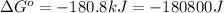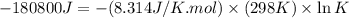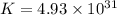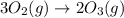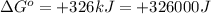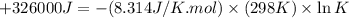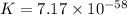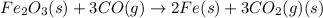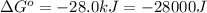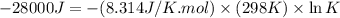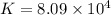Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

You know the right answer?
Which of the following reactions will have the largest value of k at 298 k? a) caco3(s) → cao(s) +...
Questions


Mathematics, 10.09.2021 01:00

Mathematics, 10.09.2021 01:00

Chemistry, 10.09.2021 01:00



Mathematics, 10.09.2021 01:00






English, 10.09.2021 01:00


Mathematics, 10.09.2021 01:00


English, 10.09.2021 01:00


English, 10.09.2021 01:00

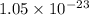
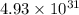
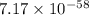
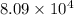
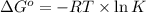
 = standard Gibbs free energy
= standard Gibbs free energy