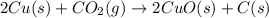The reaction 2cu(s) + co2(g) → 2cuo(s) + c(s) is endothermic. which of the following statements is true?
a) the reaction is not spontaneous at any temperature.
b) the reaction is spontaneous at all temperatures.
c) it is impossible to determine if the reaction is spontaneous without calculations.
d) the reaction will be spontaneous only at low temperatures.
e) the reaction will be spontaneous only at high temperatures.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
The reaction 2cu(s) + co2(g) → 2cuo(s) + c(s) is endothermic. which of the following statements is t...
Questions

English, 20.10.2020 01:01


Mathematics, 20.10.2020 01:01


Mathematics, 20.10.2020 01:01

Mathematics, 20.10.2020 01:01


History, 20.10.2020 01:01



English, 20.10.2020 01:01

Mathematics, 20.10.2020 01:01

Computers and Technology, 20.10.2020 01:01


History, 20.10.2020 01:01


Mathematics, 20.10.2020 01:01



English, 20.10.2020 01:01