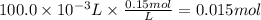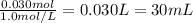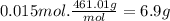Chemistry, 11.12.2019 22:31 Monaycamp13
One way to remove lead ion from water is to add a source of iodide ion so that lead iodide will precipitate out of solution: pb2+(aq) + 2i-(aq) = pbi2(s).
a. what volume of a 1.0 m ki solution must be added to 100.0 ml of a solution that is 0.15m in pb2+ ion to precipitate all the lead ion?
b. what mass of pbi2 should precipitate?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
One way to remove lead ion from water is to add a source of iodide ion so that lead iodide will prec...
Questions




History, 06.10.2019 11:00


Business, 06.10.2019 11:00




Chemistry, 06.10.2019 11:00


Mathematics, 06.10.2019 11:00





Mathematics, 06.10.2019 11:00

Mathematics, 06.10.2019 11:00


Mathematics, 06.10.2019 11:00