Chemistry, 11.12.2019 23:31 Dennismommie
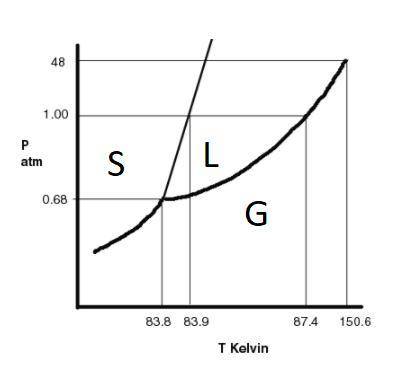
The substance argon has the following properties: normal melting point: 83.9 k normal boiling point: 87.4 k triple point: 0.68 atm, 83.8 k critical point: 48 atm, 150.6 k a sample of argon is initially at a pressure of 49.6 atm and a temperature of 101.4 k. the pressure on the sample is reduced to 0.680 atm at a constant temperature of 101.4 k. which of the following are true? choose all that apply the final state of the substance is a gas. the gas initially present will solidify. the final state of the substance is a solid. the sample is initially a liquid. one or more phase changes will occur.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

You know the right answer?
The substance argon has the following properties: normal melting point: 83.9 k normal boiling poin...
Questions



English, 21.11.2019 02:31

English, 21.11.2019 02:31


Mathematics, 21.11.2019 02:31







Mathematics, 21.11.2019 02:31


Physics, 21.11.2019 02:31

Physics, 21.11.2019 02:31

Social Studies, 21.11.2019 02:31

Biology, 21.11.2019 02:31


Mathematics, 21.11.2019 02:31