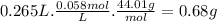Chemistry, 12.12.2019 00:31 ShlomoShekelstein
The formula that governs the concentration of gas dissolved in a solvent based on its pressure is given by henry's law c kp where: c solubility of a gas at a fixed temperature (in mol/l, m) k henry's law constant (in units of mol/l atm) p partial pressure of the gas (in units of atm).how many grams of carbon dioxide gas is dissolved in a 265 ml can of cola if the manufacturer uses a pressure of 1.7 atm in the bottling process at 25 °c? assume that the k of co2 in water = 0.034 mol/l·atm) at 25 °c. report your answer to two significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2
You know the right answer?
The formula that governs the concentration of gas dissolved in a solvent based on its pressure is gi...
Questions

Physics, 11.12.2020 06:10



Social Studies, 11.12.2020 06:10

Biology, 11.12.2020 06:10



Chemistry, 11.12.2020 06:10


Advanced Placement (AP), 11.12.2020 06:10

Biology, 11.12.2020 06:10


Mathematics, 11.12.2020 06:10

Mathematics, 11.12.2020 06:10

Mathematics, 11.12.2020 06:10


Mathematics, 11.12.2020 06:10

Chemistry, 11.12.2020 06:10

Mathematics, 11.12.2020 06:20