
An equilibrium mixture of pcl 5 ( g ) , pcl 3 ( g ) , and cl 2 ( g ) has partial pressures of 217.0 torr, 13.2 torr, and 13.2 torr, respectively. a quantity of cl 2 ( g ) is injected into the mixture, and the total pressure jumps to 263.0 torr. the appropriate chemical equation is pcl 3 ( g ) + cl 2 ( g ) − ⇀ ↽ − pcl 5 ( g ) calculate the new partial pressures after equilibrium is reestablished.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2
You know the right answer?
An equilibrium mixture of pcl 5 ( g ) , pcl 3 ( g ) , and cl 2 ( g ) has partial pressures of 217.0...
Questions

English, 27.02.2020 08:46


Social Studies, 27.02.2020 08:47

Mathematics, 27.02.2020 08:50


Mathematics, 27.02.2020 08:50

English, 27.02.2020 08:52


Mathematics, 27.02.2020 08:55


Computers and Technology, 27.02.2020 08:58

English, 27.02.2020 08:58



Computers and Technology, 27.02.2020 08:58

Biology, 27.02.2020 08:58

Social Studies, 27.02.2020 08:58



Mathematics, 27.02.2020 08:59

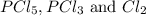 when equilibrium is re-established are 223.4 torr, 6.82 torr and 26.4 torr respectively.
when equilibrium is re-established are 223.4 torr, 6.82 torr and 26.4 torr respectively.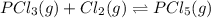
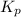 for above reaction follows:
for above reaction follows: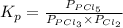 ........(1)
........(1)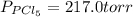
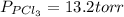
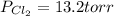
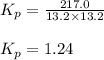
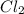 .
.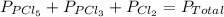
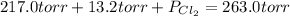
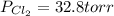
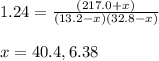
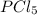 = (217.0+x) = (217.0+6.38) = 223.4 torr
= (217.0+x) = (217.0+6.38) = 223.4 torr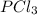 = (13.2-x) = (13.2-6.38) = 6.82 torr
= (13.2-x) = (13.2-6.38) = 6.82 torr

