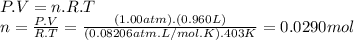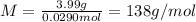Problem page a sample of an unknown compound is vaporized at . the gas produced has a volume of at a pressure of , and it weighs . assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. round your answer to significant digits. clears your work. undoes your last action. provides information about entering answers.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
Problem page a sample of an unknown compound is vaporized at . the gas produced has a volume of at a...
Questions






English, 06.01.2022 14:00



English, 06.01.2022 14:00

English, 06.01.2022 14:00


SAT, 06.01.2022 14:00

Mathematics, 06.01.2022 14:00



Mathematics, 06.01.2022 14:00



Mathematics, 06.01.2022 14:00

Mathematics, 06.01.2022 14:00