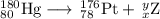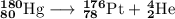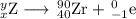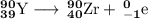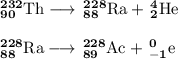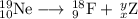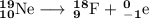Chemistry, 12.12.2019 02:31 brooke012002
Write a balanced equation for each of the following nuclear reactions: (a) mercury-180 decays into platinum-176 (b) zirconium-90 and an electron are produced by the decay of an unstable nucleus (c) thorium-232 decays and produces an alpha particle and a radium-228 nucleus, which decays into actinium-228 by beta decay (d) neon-19 decays into fluorine-19

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
Write a balanced equation for each of the following nuclear reactions: (a) mercury-180 decays into...
Questions

Advanced Placement (AP), 18.10.2020 05:01

Social Studies, 18.10.2020 05:01

Computers and Technology, 18.10.2020 05:01




Mathematics, 18.10.2020 05:01



Health, 18.10.2020 05:01

English, 18.10.2020 05:01

Social Studies, 18.10.2020 05:01

Mathematics, 18.10.2020 05:01

History, 18.10.2020 05:01





History, 18.10.2020 05:01

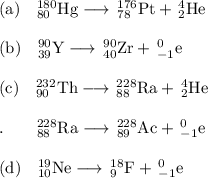
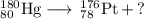
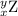 , where x = the atomic number, y = the mass number, and Z = the symbol of the element .
, where x = the atomic number, y = the mass number, and Z = the symbol of the element .