Chemistry, 12.12.2019 03:31 ayoismeisjjjjuan
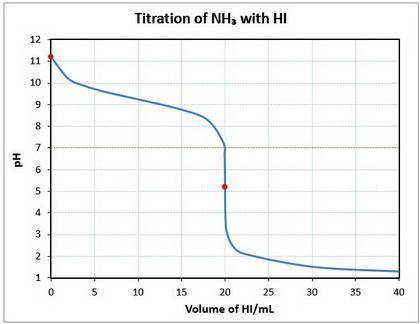
10. a 20.00 ml sample of 0.150 mol/l ammonia (nh3(aq)) is titrated to the equivalence point by 20.0 ml of a solution of 0.150 mol/l of the strong acid hydroiodic acid (hi (
a) write a balanced equation for the titration reaction.
b) what is the ph of the ammonia solution before the titration begins?
c) what is the ph at the equivalence point?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:00
An example of technology is the a. addition of a side group to an organic molecule during synthesis. b. use of a new antibiotic to fight an infection. c. measurement of iron concentration in a water sample. d. study of atomic fusion reactions
Answers: 3

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
10. a 20.00 ml sample of 0.150 mol/l ammonia (nh3(aq)) is titrated to the equivalence point by 20.0...
Questions



Mathematics, 05.05.2020 12:32

History, 05.05.2020 12:32

Mathematics, 05.05.2020 12:32

Mathematics, 05.05.2020 12:32

Mathematics, 05.05.2020 12:32


Mathematics, 05.05.2020 12:32

Social Studies, 05.05.2020 12:32

Mathematics, 05.05.2020 12:32


Mathematics, 05.05.2020 12:32



History, 05.05.2020 12:32


Mathematics, 05.05.2020 12:32


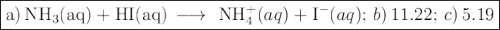

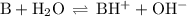
![K_{\text{b}} = \dfrac{\text{[BH}^{+}]\text{[OH}^{-}]}{\text{[B]}} = 1.8 \times 10^{-5}\\\\\dfrac{x^{2}}{0.150 - x} = 1.8 \times 10^{-5}](/tpl/images/0414/6924/2256c.png)
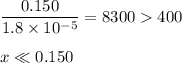
![\dfrac{x^{2}}{0.150} = 1.8 \times 10^{-5}\\\\x^{2} = 0.150 \times 1.8 \times 10^{-5}\\x^{2} = 2.7 \times 10^{-6}\\x = \sqrt{2.7 \times 10^{-6}}\\x = \text{[OH]}^{-} = 1.64 \times 10^{-3} \text{ mol/L}](/tpl/images/0414/6924/0b94c.png)
![\text{pOH} = -\log \text{[OH}^{-}] = -\log(1.64 \times 10^{-3}) = 2.78\\\\\text{pH} = 14.00 - \text{pOH} = 14.00 - 2.78 = \mathbf{11.22}\\\\\text{The pH of the solution at equilibrium is } \large \boxed{\mathbf{11.22}}](/tpl/images/0414/6924/f4986.png)
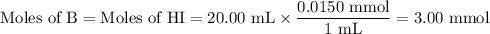
![\rm [BH^{+}] = \dfrac{\text{3.00 mmol}}{\text{40.00 mL}} = \text{0.0750 mol/L}](/tpl/images/0414/6924/607b2.png)
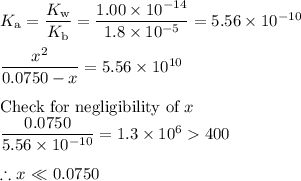
![\dfrac{x^{2}}{0.0750} = 5.56 \times 10^{-10}\\\\x^{2} = 0.0750 \times 5.56 \times 10^{-10}\\x^{2} = 4.17 \times 10^{-11}\\x = \sqrt{4.17 \times 10^{-11}}\\\rm [H_{3}O^{+}] =x = 6.46 \times 10^{-6}\, mol \cdot L^{-1}](/tpl/images/0414/6924/dc4d9.png)
![\text{pH} = -\log{\rm[H_{3}O^{+}]} = -\log{6.46 \times 10^{-6}} = \large \boxed{\mathbf{5.19}}](/tpl/images/0414/6924/b2d28.png)