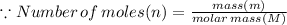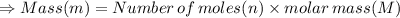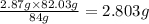Chemistry, 12.12.2019 03:31 jaylynomalley
In the stoichiometry laboratory experiment you reacted sodium bicarbonate with acetic acid to form sodium acetate, water and carbon dioxide according to the following equation. nahco3 + ch3cooh → h2o + co2 + nach3co2 calculate the theoretical yield of the reaction if you reacted 2.87 grams of sodium bicarbonate with sufficient acetic acid to produce sodium acetate. group of answer choices

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 23.06.2019 05:30
What is the body’s main processing system? it uses input from various parts to control voluntary and involutiontary movement. it’s composed of two main parts-the brain and spinal cord. a. nbs b.cns c. ans d. pns
Answers: 1

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
In the stoichiometry laboratory experiment you reacted sodium bicarbonate with acetic acid to form s...
Questions




History, 13.03.2021 03:50

English, 13.03.2021 03:50


Computers and Technology, 13.03.2021 03:50

Social Studies, 13.03.2021 03:50



Mathematics, 13.03.2021 03:50

Mathematics, 13.03.2021 03:50

Arts, 13.03.2021 03:50

Mathematics, 13.03.2021 03:50

Mathematics, 13.03.2021 03:50

Mathematics, 13.03.2021 03:50

History, 13.03.2021 03:50

Mathematics, 13.03.2021 03:50


Mathematics, 13.03.2021 03:50